Describe Diffusion Using the Words Concentration and Concentration Gradient
Similarly the medium in which diffusion occurs could also be in one of the three physical states. Diffusion is the process by which molecules spread from a region of higher concentration to a region of lower concentration.

What Is A Concentration Gradient Defination Get Education
Diffusion is driven by a gradient in Gibbs free energy or chemical potentialIt is possible to diffuse uphill from a region of lower concentration to a region of higher concentration like in spinodal decomposition.
. The role of carrier proteins and protein channels in facilitated diffusion. Diffusion is a physical process that refers to the net movement of molecules from a region of high concentration to one of lower concentration. Initially all of the red dots are within the membrane.
Writing the first law in a modern mathematical form. Ficks First Law of Diffusion. The direction of diffusion is said to be down or with the concentration gradient.
Surface area difference in concentration and the thickness of the exchange surface affect the rate of diffusion. The description of the concentration of a gradient shown in the transparency is a gradual change of solutes that are in a solution. Diffusion is a passive transport process driven by the concentration gradient at the G L interface.
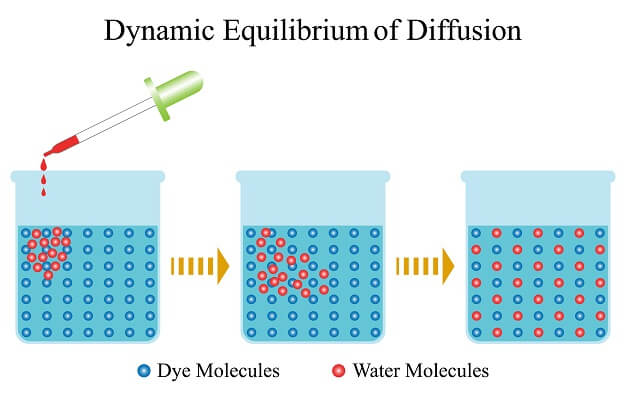
When the concentration of red dots is the same inside and outside. A concentration gradient affects the. The flux of any substance is defined as the quantity of that substance passing through a section perpendicular to.
Diffusion is the net passive movement of particles atoms ions or molecules down the concentration gradient. Molecules diffuse from areas of high concentration to areas of low concentration down a concentration gradient. The material that diffuses could be a solid liquid or gas.
How does each word of that phrase accurately describe our current. The molar flux due to diffusion is proportional to the concentration gradient. View Diffusion lab docx from BIO 111-800 at Forsyth Technical Community College.
Define isotonic hypertonic and hypotonic solutions and give examples how these 3 types of tonicity effect red blood cells and plant cells using proper terminology example. The rate of change of concentration at a point in space is proportional to the second derivative of concentration with space. Questions for Review 93 4.
Define simple diffusion using the words concentration gradient. The concentration gradient therefore represents the concept that just as a ball rolls down a slope during diffusion molecules move down the concentration gradient. The term w mosaic has been used in describing the cytoplas mic membrane.
Sketch name and describe three flagellar arrangements in bacteria. Red blood cells undergo hemolysis when placed in a hypotonic. Describe or draw an example of diffusion down a concentration gradient 2.
Higher concentration gradients will result in higher rates of diffusion. They are said to be in equilibrium if the molecules are evenly distributed. Simple diffusion is the process in which solutes are passed through the concentration gradient in a solution across a semipermeable membrane.
This allows calcium ions to enter the nerve terminal along their concentration gradient. Particles diffuse down a concentration gradient from an area of high concentration to an area of low concentration. Diffusion is the passive movement of substances down a concentration gradient.
The assistance of membrane proteins is not required in this process of diffusion. Diffusion PRE-LAB QUESTIONS 1. Diffusion is the net movement of anything for example atoms ions molecules energy generally from a region of higher concentration to a region of lower concentration.
As time passes there is net diffusion of the red dots out of the membrane following their concentration gradient. The rate of diffusion is determined by a variety of factors including the concentration gradient of the gases and the partial pressure of the gases. Diffusion stops when the concentration of the substance is equal in both areas.
Diffusion or movement of molecules happens from a high concentration to low concentration or can be said to be dependent on concentration gradient. Going down the concentration gradient means going from an area with a higher concentration of particles to an area with lower concentration of particles. Therefore chemicals diffuse across the membrane only when a concentration gradient exists across the cell membrane.
Daltons law can be used to determine the.

Concentration Gradient Examples What Is A Concentration Gradient Video Lesson Transcript Study Com

Concentration Gradient The Definitive Guide Biology Dictionary
Solved 02 Explain What Is Happening In The Following Chegg Com
0 Response to "Describe Diffusion Using the Words Concentration and Concentration Gradient"
Post a Comment